 Tyber Medical Receives 510 (k) clearance for TyFix™ All-in-One Extremity Joint Fixation Device (press release)
Tyber Medical Receives 510 (k) clearance for TyFix™ All-in-One Extremity Joint Fixation Device (press release)
Tyber Medical, LLC, a privately held company focused on developing innovative medical devices for private label opportunities and advancing the science of bioengineered surfaces, announces the 510(k) clearance for TyFix™, an all-in-one extremity joint fixation system.
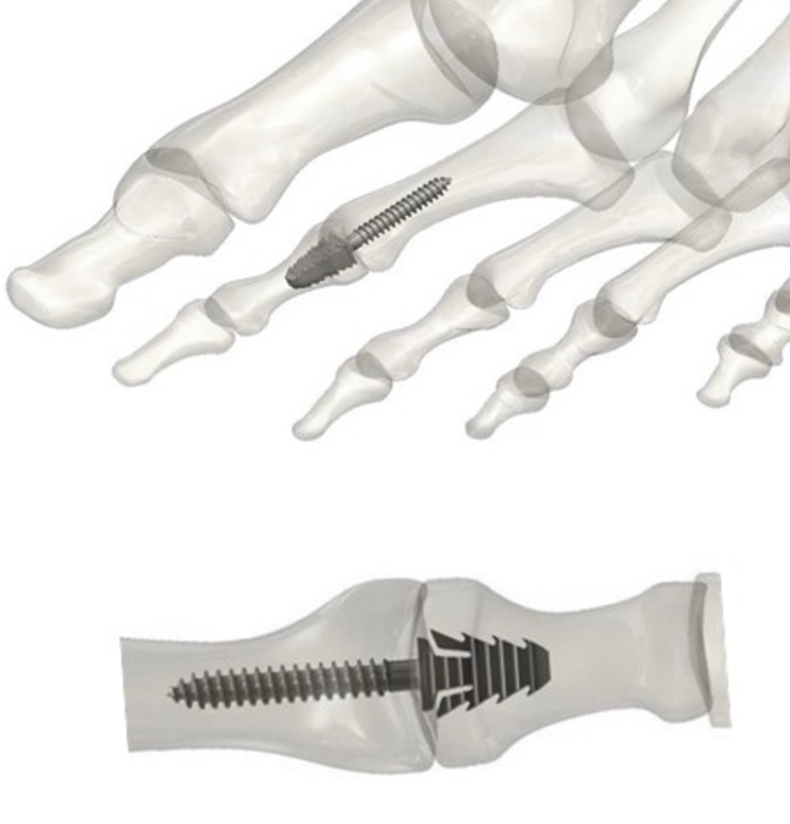
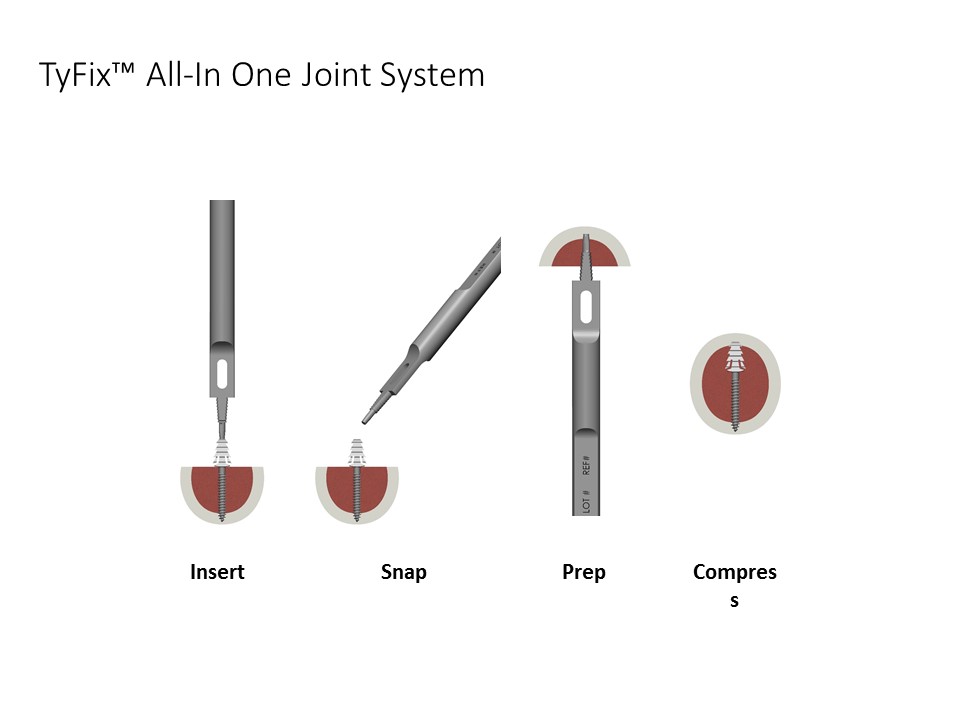
Tyber Medical is excited to announce regulatory approval for the TyFix All-in-One Extremity Joint Fixation device, an innovative system that eliminates the use of the ancillary instrumentation for a wide range of bone fixation procedures, including hammertoe correction. The integrated implant/instrument design, (first of its kind) creates value by minimalizing the number of procedure steps, improving operating room efficiency, and significantly reducing procedure time. In addition, the all-in-one sterile-packed device eliminates traditional reprocessing costs.
The TyFix Screw combines a highly optimized bone thread with a barbed head to maximize bone purchase and compression in both the proximal and distal phalanges. The system delivers intra-operative flexibility by offering solid and cannulated implants with diameters ranging from Ø1.5 – 4.0mm in multiple lengths as well as a straight and an angled 10 degree option.
“The TyFix system is the first device to fully integrate all instrumentation and implant into a unitary implant-instrument combination while also delivering a strong, robust construct, an efficient procedure, and minimal surgical time,” commented Jeff Tyber, CEO of Tyber Medical. He added, “The company continues to focus and enhance our distal extremity platform, is dedicated to accelerating the path to market for our private label customers and is always striving to add value through innovative solutions.”
The extremity market continues to experience tremendous growth and innovation. Visit Tyber Medical Booth #824 at AOFAS in Seattle, WA where the company will showcase the latest and greatest products available for private labeling.
About Tyber Medical:
Tyber Medical, LLC, Bethlehem, Pennsylvania, a rapid commercialization device company, is creating new pathways to regulatory approved bioengineered implants and instruments for orthopedic companies, large distributors, and hospital organizations. Tyber Medical designs and develops full class II orthopedic systems; verifies and validates those systems using a QSR and ISO 13485 certified quality system; and pursues and maintains both US (FDA 510k) and OUS (CE Mark) regulatory approvals. Current products include the opening wedge osteotomy system, headless and headed compression screws, snap-off screws, cervical plating system, lateral retractor system and spinal interbody spacers featuring both standard sterile and non-sterile PEEK and TyPEEK®, a proprietary titanium plasma sprayed PEEK. The company is also developing BioTy™, a nanotopography surface modification which limits the adherence of bacteria to implants. For more information, please visit www.tybermedical.com.
The TyWedge™ System and Spinal Interbody Spacers are made with PEEK-OPTIMA® from Invibio® Biomaterial Solutions.
Contact:
Eric Dickson
83 South Commerce Way, Suite 310
Bethlehem PA 18017
(866) 761-0933
[email protected]
SOURCE Tyber Medical, LLC
The TyFix Screw combines a highly optimized bone thread with a barbed head to maximize bone purchase and compression in both the proximal and distal phalanges. The system delivers intra-operative flexibility by offering solid and cannulated implants with diameters ranging from Ø1.5 – 4.0mm in multiple lengths as well as a straight and an angled 10 degree option.
“The TyFix system is the first device to fully integrate all instrumentation and implant into a unitary implant-instrument combination while also delivering a strong, robust construct, an efficient procedure, and minimal surgical time,” commented Jeff Tyber, CEO of Tyber Medical. He added, “The company continues to focus and enhance our distal extremity platform, is dedicated to accelerating the path to market for our private label customers and is always striving to add value through innovative solutions.”
The extremity market continues to experience tremendous growth and innovation. Visit Tyber Medical Booth #824 at AOFAS in Seattle, WA where the company will showcase the latest and greatest products available for private labeling.

About Tyber Medical:
Tyber Medical, LLC, Bethlehem, Pennsylvania, a rapid commercialization device company, is creating new pathways to regulatory approved bioengineered implants and instruments for orthopedic companies, large distributors, and hospital organizations. Tyber Medical designs and develops full class II orthopedic systems; verifies and validates those systems using a QSR and ISO 13485 certified quality system; and pursues and maintains both US (FDA 510k) and OUS (CE Mark) regulatory approvals. Current products include the opening wedge osteotomy system, headless and headed compression screws, snap-off screws, cervical plating system, lateral retractor system and spinal interbody spacers featuring both standard sterile and non-sterile PEEK and TyPEEK®, a proprietary titanium plasma sprayed PEEK. The company is also developing BioTy™, a nanotopography surface modification which limits the adherence of bacteria to implants. For more information, please visit www.tybermedical.com.
The TyWedge™ System and Spinal Interbody Spacers are made with PEEK-OPTIMA® from Invibio® Biomaterial Solutions.
Contact:
Eric Dickson
83 South Commerce Way, Suite 310
Bethlehem PA 18017
(866) 761-0933
[email protected]
SOURCE Tyber Medical, LLC