 Simplify Medical raises $21m for MR-safe cervical disc (MassDevice)
Simplify Medical raises $21m for MR-safe cervical disc (MassDevice)
Simplify Medical said today that it plans to use its $21 million Series B round to complete a pair of pivotal studies for its namesake cervical disc replacement
The round was led by Life Sciences Partners, Sunnyvale, Calif.-based Simplify said, joined by new backer Sectoral Asset Management and M.H. Carnegie, the Australian private equity shop that acquired Simplify in March 2015.
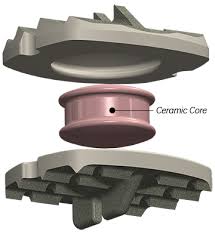
The Simplify device is designed to be safe during magnetic resonance imaging scans, in that it’s designed to eliminate the imaging artifact created by the metals used in some artificial disc replacements by using a PEEK-on-ceramic construction. The aim is to eliminate the need for computed tomography scans after implantation and the attendant patient exposure to ionizing radiation. The Simplify disc won CE Mark approval in the European Union in February 2015.
Simplify said today that the proceeds are earmarked for pivotal work studying the implant in single-level and two-level cervical fusion procedures.
“We are gratified by the confidence investors are showing in our Simplify disc,” CEO David Hovda said in prepared remarks. “The new funds will enable us to develop the rigorous evidence that gets us one step closer to availability for U.S. patients in need.”
“We are very impressed with the intelligence of the Simplify disc technology,” added LSP general partner Fouad Azzam. “With hospitals being held to higher standards relative to complications and post-operative costs, it is important that innovations minimize patient risk. Not only does the Simplify disc avoid substantial radiation exposure, it also avoids metal wear that has been problematic for other orthopedic devices. In addition, it offers the lowest-profile device available, opening up a broader patient population for the technology.”